Clinical Anatomy
Parkinson’s Disease is considered predominantly a disorder of the basal ganglia[1].
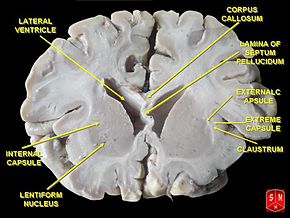
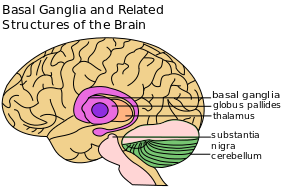
The basal ganglia are a group of nuclei situated deep and centrally at the base of the forebrain. They have robust connections with the cerebral cortex and thalamus in addition to other areas of the brain. Their vast system of communication allows them involvement with a variety of functions, including automatic and voluntary motor control, procedural learning relating to routine behaviors and emotional functions. The association with other cortical areas ensures smoothly orchestrated movement control and motor behaviour.
The striatum, composed of the caudate and putamen, is the largest nuclear complex of the basal ganglia. The striatum receives excitatory input from several areas of the cerebral cortex, as well as inhibitory and excitatory input from the dopaminergic cells of the substantia nigra pars compacta (SNc). These cortical and nigral inputs are received by the spiny projection neurons, which are of 2 types:
- Those that project directly to the internal segment of the globus pallidus (GPi), the major output site of the basal ganglia. The consequence of this pathway is to increase the excitatory drive from thalamus to cortex i.e. as the motor cortex increases firing rates, this results in increased activity in the corticospinal tract and eventually the muscles, so ‘turns up’ the action of the motor system.
- Those that project to the external segment of the globus pallidus (GPe), establishing an indirect pathway to the GPi via the subthalamic nucleus (STN). The consequence of the indirect pathway is to decrease the excitatory drive from thalamus to cortex. The increase in inhibition of the thalamic neurons in effect ‘turns down’ motor activity from the cortex
The actions of both pathways regulate the neuronal output from the GPi, thus providing tonic inhibitory input to the thalamic nuclei that project to the primary and supplementary motor areas.
Get to know more about the basal ganglia
Here are some sources of information about the anatomy and function of the basal ganglia:
- Pathology
The aetiology of Parkinson’s is still unclear, with hypotheses as diverse as a peripheral versus central nervous system origin, intrinsic cellular oscillator versus network oscillators, and basal ganglia-based pathophysiology versus cerebellar-thalamic based pathophysiology[3].
Parkinson’s is traditionally defined, pathologically, by the finding of Lewy bodies and degeneration of catecholaminergic neurones at post-mortem[4]. Using a pathological definition however is problematic as it is not practical in life. The two recognised neuro-pathological findings in the brains of people with Parkinson’s are:- Loss of pigmented dopaminergic neurons of the substantia nigra pars compacta[5]. The loss of dopamine neurons occurs most prominently in the ventral lateral substantia nigra . Approximately 60-80% of dopaminergic neurons are lost before the motor signs of Parkinson’s emerge. Dopamine depletion is believed to result from degeneration of dopamine nigrostriatal system, antipsychotic drugs, toxic reactions to chemical agents or poisoning[6].
- The presence of Lewy bodies and Lewy neuritis. Some individuals who were thought to be normal neurologically at the time of their deaths are found to have Lewy bodies on autopsy examination. These incidental Lewy bodies have been hypothesised to represent the pre-symptomatic phase of Parkinson. Their prevalence increases with age, but they are specific to Parkinson’s and are found in some cases of atypical parkinsonism and other disorders.
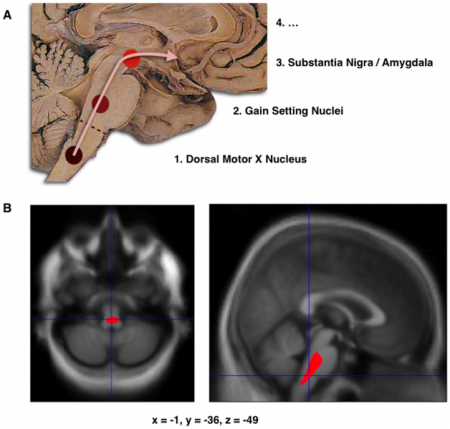
A. Schematic initial progression of Lewy body deposits in the first stages of Parkinson’s Disease, as proposed by Braak and colleagues[7].
B. Localization of the cluster of significant volume reduction in PD compared with people without the disease. The significant cluster located in the medulla oblongata/pons is superimposed as a red blob on the mean normalized anatomical scan of all participants.
Prognosis[8]
Prior to Levodopa
Before the introduction of levodopa, Parkinson caused severe disability or death in 25% of patients within 5 years of onset, 65% within 10 years, and 89% within 15 years. The mortality rate from Parkinson was 3 times that of the general population matched for age, sex, and racial origin.
Post Levodopa
With the introduction of levodopa, the mortality rate dropped approximately 50%, and longevity was extended by many years. This is thought to be due to the symptomatic effects of levodopa, as no clear evidence suggests that levodopa stems the progressive nature of the disease.
Factors affecting prognosis:
The American Academy of Neurology notes that the following clinical features may help predict the rate of progression of Parkinson’s:
- Older age at onset and initial rigidity/hypokinesia can be used to predict (1) a more rapid rate of motor progression in those with newly diagnosed Parkinson disease and (2) earlier development of cognitive decline and dementia; however, initially presenting with tremor may predict a more benign disease course and longer therapeutic benefit from levodopa
- A faster rate of motor progression may also be predicted if the patient is male, has associated comorbidities, and has postural instability/gait difficulty (PIGD)
- Older age at onset, dementia, and decreased responsiveness to dopaminergic therapy may predict earlier nursing home placement and decreased survival
Get to know more about the pathophysiology of Parkinson’s:
- Bergman H, Deuschl G (2002). Pathophysiology of Parkinson’s disease: From clinical neurology to basic neuroscience and back. Movement Disorders; 17; Suppl 3:S28-40
- Diagnosis
Idiopathic Parkinson’s has been divided into three clinical stages:
1. Pre-clinical stage: disease can predicted or early detected through biomarkers such as blood tests, saliva and/or genetic markers. In this stage, people with risk factors could be identified and triaged for early intervention.
2. Prodromal Parkinson’s: this stage is characterized by the presence of symptoms such as decreased sens of smelling (hyposmia), low mood/ depression. bowel symptoms and/or sleep changes.
3. Clinical Parkinson’s: symptoms defined by dopamine-responsive motor features; bradykinesia (with a large scale of sensory motor changes symptoms and symptoms and fatigue), tremors and /or rigidity.
There are no specific, standard criteria to diagnose Parkinson’s because the specificity and sensitivity of its characteristics are hard to clearly establish. The aetiology is unclear and most cases are hypothesised to be due to a combination of genetic and environmental factors. In most people, the diagnosis of Parkinson’s is based on clinical findings[10].
The most widely accepted clinical criteria for the diagnosis of PD are those introduced by the United Kingdom Parkinson’s Disease Society Brain Bank Diagnostic Criteria for Parkinson’s Disease:
Step 1: Diagnosis of Parkinsonism
- Bradykinesia and at least one of the following:
- Muscular rigidity
- 4–6 Hz resting tremor
- Postural instability not caused by primary visual, vestibular, cerebellar or proprioceptive dysfunction. it is marker of parkinson’s but no essentially a diagnostic criteria. it could be due to many other conditions.
Step 2: Features tending to exclude Parkinson’s as the cause of Parkinsonism
- History of repeated strokes with stepwise progression of parkinsonian features
- History of repeated head injury
- History of definite encephalitis
- Neuroleptic treatment at onset of symptoms
- >1 affected relatives
- Sustained remission
- Strictly unilateral features after 3 years
- Supranuclear gaze palsy
- Cerebellar signs
- Early severe autonomic involvement
- Early severe dementia with disturbances of memory, language and praxis
- Babinski’s sign
- Presence of a cerebral tumour or communicating hydrocephalus on computed tomography scan
- Negative response to large doses of levodopa (if malabsorption excluded)
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) exposure
Step 3: Features that support a diagnosis of Parkinson’s (three or more required for diagnosis of definite Parkinson’s disease)
- Unilateral onset
- Rest tremor present
- Progressive disorder
- Persistent asymmetry affecting the side of onset most
- Excellent (70–100%) response to levodopa
- Severe levodopa-induced chorea
- Levodopa response for ≥5 years
- Clinical course of ≥10 years
- In recent years, attempts to define Parkinson’s genetically have become possible with the discovery of monogenic forms of the condition. Currently known genetic causes of Parkinson disease account for approximately 10% of cases.
Role Of PET Scans in Diagnosis
Scanning for loss of dopamine, the major chemical responsible for some symptoms of Parkinson’s, permits comparison against scans of people who have no dopamine deficiency. By itself, a scan does not make the diagnosis of Parkinson’s but identifies the decline in dopaminergic neurones. It is used by experienced neurologists in cases where the diagnosis of Parkinson’s is uncertain.
Scans include radionuclide positron emission tomography (PET) or by injecting radiopharmaceutical agent (used to detect the presence of dopamine transporters (DaT) in the brain) into a patient’s veins in a procedure referred to single photon emission computed tomography (SPECT) imaging.PET scans typically focus on glucose (sugar) metabolism, and DaT/SPECT scans focus on the activity of the dopamine transporter. Unfortunately, this decline is seen in conditions other than Parkinson’s such as Multiple Systems Atrophy (MSA) and Progressive supranuclear palsy (PSP).
Value of Accurate Diagnosis
An accurate diagnosis in a person with suspected Parkinson’s has an important bearing on prognosis – especially in those with younger onset diagnosis[11]. People with Parkinson’s will have a longer life expectancy than those with MSA or PSP and will respond better to dopaminergic medication. It should also be differentiated from other conditions presenting with tremor such as a postural and action tremor similar to that seen in essential tremor. Other causes of Parkinsonism and Parkinson’s-Plus syndromes include multiple cerebral infarction. Differential diagnosis can also be difficult in elderly people since extrapyramidal symptoms and signs are common.
Factors predicting Parkinson’s
A 2014 study by Schrag et al aimed to identify motor and non-motor features present before the diagnosis of Parkinson’s disease.
Tremor, balance impairments, constipation, hypotension, erectile dysfunction, urinary dysfunction, dizziness, fatigue, depression and anxiety were present with higher rates in patients 5 years before their diagnosis with Parkinson’s compared to controls. Constipation, which is believed to be one of the early diagnostic features of Parkinson’s, was found to be higher at 10 years before the diagnosis. Higher incidents of memory problems were reported 2 years before the diagnosis[12].
Related pages
References
- ↑ Obeso JA, Rodríguez-Oroz MC, Benitez-Temino B, et al. (2008). “Functional organization of the basal ganglia: therapeutic implications for Parkinson’s disease”. Mov. Disord. 23 (Suppl 3): S548–59.
- ↑ Mike Ravenek. Basal Ganglia Motor Circuit – Function & Dysfunction. Available from: ↑ Bergman H, Deuschl G. Pathophysiology of Parkinson’s disease: from clinical neurology to basic neuroscience and back. Mov Disord. 2002;17 Suppl 3:S28-40.
- ↑ Dickson DV (2007). “Neuropathology of movement disorders”. In Tolosa E, Jankovic JJ. Parkinson’s disease and movement disorders. Hagerstown, MD: Lippincott Williams and Wilkins. pp. 271–83
- ↑ Dickson DV (2007). “Neuropathology of movement disorders”. In Tolosa E, Jankovic JJ. Parkinson’s disease and movement disorders. Hagerstown, MD: Lippincott Williams and Wilkins. pp. 271–83
- ↑ Cns basal ganglia. Avialable from: https://www.slideshare.net/physiologylectures/cns-basal-ganglia
- ↑ Braak H, Del Tredici K, et al. (2003). “Staging of brain pathology related to sporadic Parkinson’s disease.”. Neurobiol Aging 24 (2): 197–211.
- ↑ Robert A Hauser; Selim R Benbadis. ↑ Armando Hasudungan. Neurodegenerative Disorder II – Parkinson’s and Huntington’s. Available from: ↑ Jankovic J (April 2008). “Parkinson’s disease: clinical features and diagnosis”. J. Neurol. Neurosurg. Psychiatr. 79 (4): 368–76.
- ↑ Prevalence and relation of dementia to various factors in Parkinson’s disease.fckLRRana AQ,et al.Psychiatry Clin Neurosci. 2012 Feb;66(1):64-8.
- ↑ Schrag A, Horsfall L, Walters K, Noyce A, Petersen I. Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. The Lancet Neurology. 2015 Jan 1;14(1):57-64.