Introduction
A wide range of treatment techniques and approaches from different philisophical backgrounds are utilised in Neurological Rehabilitation. Research to support the different approaches varies hugely, with a wealth of research to support the use of some techniques while other approaches have limited evidence to support its use but rely on ancedotal evidence. This page provides a brief overview of some of the approaches used in Stroke Rehabilitation with evidence based clinical guideline recommendations.
State of the Evidence
The past decade has seen an exponential growth in the number of Randomised Control Trials in relation to physiotherapy interventions utilised in Stroke. Veerbeek et al (2014) highlight that the number of RCTs on Stroke Interventions has almost quadrupled in the past 10 years, with strong evidence seen in 30 out of 53 interventions for beneficial effects on one or more outcomes. They suggest the main change lies in the increased number of interventions to which ‘strong evidence’ could be assigned and an increase in the number of outcomes for which the findings are statistically significant. [1]
Higher intensity of practice appears to be an important aspect of effective physical therapy and suggestion is that intensity of practice is a key factor in meaningful training after stroke, and that more practice is better. 17 hours of therapy over a 10 week period has been found to be necessary for significant positive effects at both the body function level as well as activities and participation level of the ICF. Yet despite the fact that National Clinical Guidelines advocate for at least 45 mins of therapy daily as long as there are rehabilitation goals and the patient tolerates this intensity, and recognition that high-intensity practice is better there still remains a big contrast between the recommended and actual applied therapy time. Recent surveys in the Netherlands showthat patients with stroke admitted to a hospital stroke unit only received a mean of 22 minutes of physical therapy on weekdays. Similarly, in the United Kingdom inpatients received 30.6 minutes physical therapy per day. Both of these significantly fall short of the recommended 45 mins daily. [1]
While the body of knowledge in relation to physiotherapy in stroke rehabilitation is still growing further confirmation of the evidence for physiotherapy after stroke, and facilitating the transfer to clinical practice, requires a better understanding of the neurophysiological mechanisms, including neuroplasticity, that drive stroke recovery, as well as the impact of physiotherapy interventions on these underlying mechanisms. Similarily further research is required to support physiotherapy implementation strategies in order to optimize the transfer of scientific knowledge into clinical practice.
High growth in evidence does in its own way create challenges for physiotherapists in keeping up to date with new evidence as it becomes available and there is a need for further investigation into more effective and efficient methods for physiotherapists to keep their knowledge and skill level up-to-date in the long term.[1]
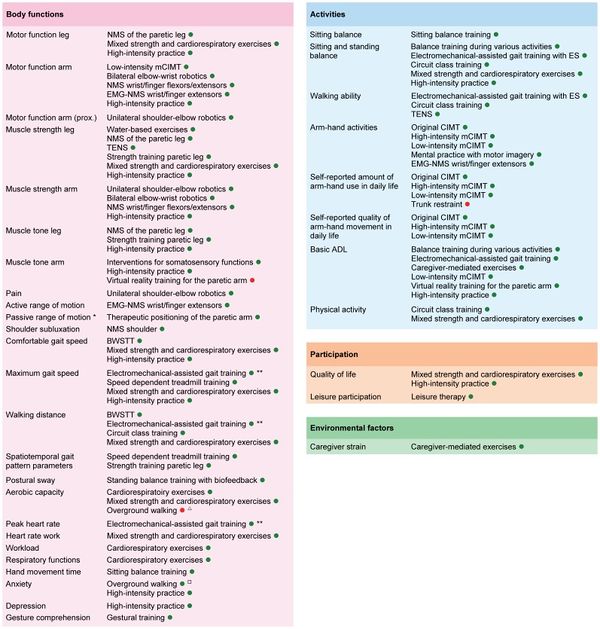
The following Fig.1 graphically displays therange of outcomes classified according to the ICF, with corresponding interventions for which is strong evidence that they significantly affect those outcomes. It should be noted that the clinical applicability of some interventions like electromechanical-assisted gait training and robot-assisted arm training is questionable, due to the accompanying high costs of the equipment. For these interventions, there are often alternative ‘strong evidence’ interventions available. [1]
Figure 1. Overview of Outcomes for which Interventions are available with Significant Summarized Effects
This graphically displays the outcomes classified according to the ICF, with corresponding interventions for which is strong evidence that they significantly affect those outcomes. Legend: A green point indicates that the intervention has a significant positive effect on the outcome, while a red point indicates that the intervention has a significant negative effect on the outcome; [1]
Interventions
Positioning
Ability to change position and posture is affected in many individuals post stroke as a result of varying degrees of physical impairments. Therapeutic positioning aims to reduce skin damage, limb swelling, shoulder pain or subluxation, and discomfort, and maximise function and maintain soft tissue length. It is also suggested that positioning may assist in reduction of respiratory complications such as those caused by aspiration and avoid compromising hydration and nutrition. The aim of positioning the patient is to try to promote optimal recovery and comfort by modulating muscle tone, providing appropriate sensory information, increasing spatial awareness, improved ability to interact with the environment and prevention of complications such as pressure sores, and contracture. [1] [2] [3]
The most appropriate position in which to place a patient following a stroke remains unclear. There is no Randomised Control Trial evidence to support the recommendation of any one position over another but five main positions have been recommened, a survey of physiotherapists’ current positioning practices found the most commonly recommended positions to be: sitting in an armchair as recommended by 98% of respondents; side lying on the unaffected side then side lying on the affected side. Sitting in a wheelchair (78%, 95% CI 74 to 82%) and supine lying were less commonly recommended. [4]
Practice Statement
Consensus-based Recommendation
- Initial specialist assessment for positioning should occur in acute stroke as soon as possible and where possible within 4 hours of arrival at hospital.
- Arm Support devices such as a Lap Tray may be used to assist with arm positioning for those at risk of shoulder subluxation
- Education and training around correct manual handling and positioning should be provided to the individual with stroke, their family/carer and health professionals, particularly nursing and other allied health staff. [5]
- Elevation of the limb when resting should be considered for individuals who are immobile to prevent swelling in the hand and foot. [6]
Early Mobilisation
Immobility is associated with a number of post stroke complications such as deep vein thrombosis etc. Early mobilization aims to reduce the time that elapses between stroke and the first time the patient leaves the bed, increasing the amount of physical activity that the patient engages in outside of bed. Early mobilisation (e.g. activities such as sitting out of bed, transfers, standing and walking) aims to minimise the risk of the complications of immobility and improve functional recovery[7]. There remains some ongoing discssion about the exact meaning of very early mobilization but Verbeek et al (2014) define early mobilization as ‘mobilizing a patient out of bed within 24 hours after the stroke, and encouraging them to practice outside the bed’ [8] [4]. Recent changes in recommendations have been made as a result of the AVERT Trial RCT of over 2000 individuals with acute stroke, which showed that very early, more frequent, higher dose mobilisation focused on out-of-bed activities in addition to usual care was worse than usual care alone and led to greater disability at three months with no effect on immobility-related complications or walking recovery [7].
Strong Recommendation FOR
- Patients with difficulty moving after stroke should be assessed as soon as possible within the first 24 hours of onset by an appropriately trained healthcare professional to determine the most appropriate and safe methods of transfer and mobilisation. [7]
- Commence mobilisation (out of bed activity) within 24 – 48 hrs of stroke onset unless receiving palliative care.[5] [7]
Strong Recommendation AGAINST
Balance
Balance difficulties are commone for many individuals post stroke usually due to a combination of reduced limb and trunk motor control, altered sensation and sometimes centrally determined alteration in body representation such that the person misperceives their posture in relation to the upright. Impaired balance often leads to reduced confidence, fear of falling and increases the risk of falls. Current evidence suggests that trunk exercise training improve trunk performance and dynamic sitting balance [9], while task specific training improves dynamic balance in both sitting and standing.[10] [7] [5] [1]
Sitting
Strong Recommendation
Practising reaching beyond arm’s length while sitting with supervision/assistance should be undertaken for individuals who have difficulty with sitting. [9]
Standing
Strong Recommendation
Practice of standing balance should be provided for individuals who have difficulty with standing. Strategies could include:
- Practising functional task-specific training while standing [11] [1] [12] [10]
- Walking training that includes challenge to standing balance (e.g. overground walking, obstacle courses) [12]
- Providing visual or auditory feedback [5] [1] [13]
- Receive progressive balance training
- Recieve lower limb strengthening exercises [7]
- Consider for an ankle-foot orthosis [14]
Gait & Mobility
The highest priority for many people with limited mobility after stroke is to walk independently. This section focuses on treatments and equipment aimed at improving walking and includes exercise. Individuals post stroke benefit from time spent in task-specific, walking-orientated leg exercises which have a cardiorespiratory focus both early and late after stroke. Interventions should be of a sufficient intensity with a focus on progression, task-specificity and challenge to improve outcomes and can include strengthening exercises for the leg, over-ground walking, circuit classes and treadmill training with and without body weight support [7]. If walking performance is poor after stroke, community activity may be limited and people may become housebound and isolated from society.
Strong Recommendation
Tailored repetitive practice of walking (or components of walking) should be practiced as often as possible for individuals with difficulty walking. The following modalities can be used to achieve this:[5]
Weak Recommendation
Other interventions may be used in addition to those above:
Treadmill Training
Treamill training can be utilised for both Gait Re-education / Training but also to aid improvements in aerobic function. Treadmill training can be completed with the patient’s body-weight partially supported by a harness in order to grade the amount of body weight supported, which is used for individuals with significant functional limitations. Speed dependent treadmill training without a harness may also be utilised. Therapists facilitate alternating stepping and weight-bearing, and as many as three therapists may be required to assist with the complete gait cycle. Shepherd and Carr [23] argued that there are three reasons why treadmill training can support gait re-education:
- It allows a complete practice of the gait cycle
- It provides opportunity for gaining improvements in speed and endurance
- It optimises aerobic fitness
Task-specific training on a treadmill has also been shown to induce expansion of subcortical and cortical locomotion areas in individuals following stroke and can result in an increase in cadence and a shortening of step length as compared to overground walking. Treadmill training may improve walking speed and endurance however it does not appear to be more effective than other walking-orientated interventions of matched intensity for improving walking ability. [23] [7] [16]
Moderate Recommendation
- People who are able to walk independently after stroke should be offered treadmill training with or without body weight support or other walking-orientated interventions at a higher intensity than usual care and as an adjunct to other treatments. [7] (Level 1)
Electromechanical Assisted
Electromechanical-assisted gait training, with and without partial body weight support as well as with or without FES, are used as adjuncts to overground gait training for the rehabilitation of patients after stroke and can be used to give non-ambulatory patients intensive practice (in terms of high repetitions) of complex gait cycles. Automated electromechanical gait machines consist either of a robot-driven exoskeleton orthosis or an electromechanical solution with two driven foot-plates simulating the phases of gait aand offer reduced effort for therapists, as they no longer need to set the paretic limbs or assist trunk movements. The main difference between electromechanical-assisted and treadmill training is that the process of gait training is automated and supported by an electromechanical solution. Current research indicates that repetitive gait training in combination with physiotherapy may improve walking ability in patients after stroke.[24]
Moderate Recommendation
- People who cannot walk independently after stroke should be considered for electromechanical-assisted gait training including body weight support. [7] (Level 1)
Rhythmic Cueing
Motor Control research provides considerable evidence that auditory rhythm can improve timing and variability of motor responses, specifically, in motor tasks with complex timing requirements or in disorders affecting timing of movement, external rhythm can provide additional stability to timekeeper mechanisms in the brain. [25]
For Rhythmic Cueing the patient’s steps are matched to the beat of the metronome or specially prepared music in order to synchronise motor responses into stable time relationships. The patient is asked to take steps according to the beat, so the rhythmic beat acts as a cue. If the beats are of a consistent frequency, this cueing will promote the temporal symmetry of walking. If the frequency of these consistent beats is increased, cadence and, therefore, speed will also increase. [26] The goal is to influence gait parameters such as pace frequency, stride length and hence walking speed and symmetry. [8]
Systematic review provides evidence that gait training with cueing of cadence is more effective than gait training alone in improving walking after stroke. Gait training with cueing of cadence produced faster walking and longer stride length, and may have positive effects on cadence and symmetry. [26]
Moderate Recommendation
Virtual Reality
Advances in virtual reality technology mean that devices using computer and gaming technology, such as the Nintendo Wii ®, are now found in many people’s homes. The potential of these types of adjuncts to maximize task-orientated practice and increase energy expenditure are beginning to be explored. Virtual reality mobility training involves the use of computer technology which enables the patient to move about in a virtual environment and receive feedback on their performance and is suggested that the use of a virtual environment produces cortical reorganization. Furthermore, virtual environments are adaptable and can afford patients the opportunity to practice under a variety of simulated circumstances. [8]
The difficulty level of the training scenarios can be adjusted by varying the speed and slope of the treadmill, the complexity of tasks, and the amount of body weight support and can allow immediate patient feedback on performance, which is an important component of learning. While skilled therapy will always be a part of rehabilitation, the use of VR-enhanced treadmill training may be a cost-effective way to increase patient motivation to practice walking under different simulated conditions.
It remains unclear whether virtual reality mobility training is more effective than other interventions for patients with a stroke in terms of comfortable and maximum walking speed, spatiotemporal gait parameters and walking ability.
Moderate Recommendation
- Virtual Reality Training can be utilised in addition to conventional gait training. [5]
Overground Walking
Overground waliking involves walking and walking-related activities on a solid surface, where the physiotherapist observes the patient’s gait, usually on a level surface, and has the patient do a range of differnt activities and exercises to influence their gait. The benefit is that over- ground gait training can be used in almost any setting or location without requiring a great deal of high-tech equipment. [8]
Moderate Recommendation
- It has been demonstrated that overground gait training by stroke patients who are able to walk without physical support is more effective in increasing walking distance and reducing anxiety than walking on a treadmill. (Level 1) [8]
Weak Recommendation
- Individualised goals should be set and assistance with adaptive equipment, information, and further referral on to other agencies should be provided for individuals who have difficulty with outdoor mobility in the community.
- Walking practice may benefit some individuals and if provided, should occur in a variety of community settings and environments, and may also incorporate virtual reality training that mimics community walking. [27] [5]
Orthotics
Orthotics, like any tool used in the treatment of a complex and chronic condition, can target all levels of health at once. It may be an intervention designed to change body structures, or an intervention to support and stabilize unresponsive mus- cles so an activity can be performed, or an adjunct to enable participation in a life role such as work. According to Leonard et al (1989) an orthosis is a device that, when applied correctly to an appropriate external surface of the body will achieve one of more of the following: [28][29]
- Relief of Pain
- Immobilisation of Musculoskeletal Segments
- Reduced Axial Loading
- Prevention or Correction of Deformity
- Imprived Function
Orthoses are made from various types of materials including thermoplastics, carbon fibre, metals, elastic, EVA, fabric or a combination of similar materials. Some designs may be purchased at a local retailer; others are more specific and require a prescription from a physician, who will fit the orthosis according to the patient’s requirements. Over-the-counter braces are basic and available in multiple sizes. They are generally slid on or strapped on with Velcro, and are held tightly in place. [29]
KNGF Clinical Guidelines recommends trial of Ankle Foot Orthotic for patients whose safe and/or efficient walking ability is impeded by drop foot during the swing phase of walking following Multidisciplinary consultation. [8]
Weak Recommendation
Upper Limb
Up to 85% of indviduals post stroke experience altered arm function, with approximately 40% of individuals being affected by upper limb function long term. Loss of arm function adversely affects quality of life, and functional motor recovery in affected upper extremities in patients with hemiplegia is the primary goal of physical therapists. “Currently there is no high quality evidence for any interventions that are routine practice, and evidence is insufficient to enable comparison of the relative effectiveness of interventions.” In other words, the evidence is insufficient to show which of the interventions are the most effective for improving upper limb function” [7].
Strong Recommendation
- People with stroke with potential or actual arm movement should be given every opportunity to practice functional activities that incorporate movements that are of high intensity, repetitive and are task-specific. These activities may be bilateral or unilateral depending on the task. [7]
Bilateral Arm Training
Bilateral Arm Training provides intensive training of bilateral coordination to enable practice of bimanual skills. During bilateral arm training, movement patterns or activities are performed with both hands simultaneously but independent from each other and can also be cyclic. This approach was developed in response to identified limitations of Constraint Induced Movement Therapy (CIMT) which precludes the opportunity to practice bilateral skills particularly functional activities that are inherently bimanual. Unilateral and bilateral training are similarly effective. However, intervention success may depend on severity of upper limb paresis and time of intervention post-stroke.
Weak Recommendation
Constraint Induced Movement Therapy
Constraint-induced movement therapy (CIMT) involves intensive targeted practice with the affected limb while restraining the non-affected limb, which means that during task-specific practice, individuals with hemiplegic stroke are forced to use their affected limb. Following a neurological incident, often the affected arm and hand are not used sufficiently even if there is some functional activity present. To address this ‘Learned non-use’, the approach of CIMT was developed whereby the non-affected limb was constrained hereby forcing the affected limb to work. This forced-use therapy combined with shaping and goal-directed training is now commonly known as CIMT. [34] [35]
Different categories of CIMT can be distinguished for use in Stroke depending on the duration of the immobilization of the paretic arm and the intensity of task-specific practice: [1]
Original CIMT Applied for 2 to 3 weeks consisting of immobilization of the non-paretic arm with a padded mitt for 90% of waking hours utilising task-oriented training with a high number of repetitions for 6 hours a day; and behavioral strategies to improve both compliance and transfer of the activities practiced from the clinical setting to the patient’s home environment.
High-intensity mCIMT Consists of immobilization of the non-paretic arm with a padded mitt for 90% of waking hours with between 3 to 6 hours of task-oriented training a day. Found to be more beneficial in the acute stage pf rehabilitation with less effect on chronic upper limb impairment.
Low-intensity mCIMT Consisted of immobilization of the non-paretic arm with a padded mitt for > 0% to
Strong Recommendations
- Intensive Constraint Induced Movement Therapy (minimum 2 hours of active therapy per day for 2 weeks, plus restraint for at least 6 hours a day) should be provided to improve arm and hand use for individuals with 20 degrees of active wrist extension and 10 degrees of active finger extension. [36] [7] [5]
- Trunk restraint may also be incorporated into the active therapy sessions at any stage post-stroke. [37] [5]
Electrical Stimulation
Functional Electrical Stimulation appears to moderately improve upper limb activity compared with both no intervention and training alone. Current evidence suggest that electrical stimulation should be used in stroke rehabilitation to improve the ability to perform functional upper limb activities.
Strong Recommendation
Robot Assisted Arm Training
Robot-mediated treatment utilises automated devices to provide passive, active or resistive limb movement which could allow for extended periods of treatment and treatments that are responsive to the particular needs of the individual by using the person’s movement as feedback, as ability changes over time. There is currently conflicting evidence as there is still limited evidence to suggest when or how often robot assisted arm movement should be used. People with reduced arm function after a stroke should only be offered robot-assisted movement therapy or neuromuscular electrical stimulation as an adjunct to conventional therapy in the context of a clinical trial. [39] [7]
Strong Recommendation
- Robot /Mechanical assisted arm training should be used to improve upper limb function in indivduals with mild to severe arm weakness after stroke “as an adjunct to conventional therapy in the context of a clinical trial”. [5]
Virtual Reality
The evidence base for virtual reality and interactive video gaming-based interventions for the arm (as an adjunct to usual care to increase overall therapy time) is developing, though studies are often of low quality and further research is needed.
Strong Recommendation
Mirror Therapy
Weak Recommendation
Mental Practice
Motor imagery (MI) is a mental process of rehearsal for a given action in order to improve motor function while Mental Practice (MP) is a training method during which a person cognitively rehearses a physical skill using MI in the absence of overt, physical movements for the purpose of enhancing motor skill performance. Mental practice with motor imagery ‒ the practising of movements and activities ‘in the mind’ ‒ has been advocated to aid recovery following stroke. Current Systematic Reviews support the use of mental practice as an adjunct to conventional therapy techniques for arm rehabilitation in the acute, sub-acute and chronic phases of stroke, particulalry in those with more severe impairment of the upper limb. [42] Interestingly, Mental Practice using Motor Imagery increases affected arm use, thus overcoming this movement suppression phenomenon and combined with Physical Practice training was better for the restoration of hand function than Physical Practice alone.
Weak Recommendation
Splinting
Strong Recommendation AGAINST
Cardiorespiratory Training
There is an increasing range of aerobic exercise options being accessed by people with following Stroke. These range from aerobic exercise programmes (e.g. overground walking or treadmill training programmes) and an array of sporting and exercise classes to the use of technology (e.g. virtual reality training). These options, supported by the growing body of evidence, present the therapist and patient with the ability to select a programme for an individual, which is timely and can be carried out in an appropriate environment.
Strong Recommendation
- Rehabilitation should include individually tailored exercise interventions to improve cardiorespiratory fitness.[5]
Practice Statement
Consensus-based Recommendations
- Commence cardiorespiratory training during their inpatient stay.
- Encourage to participate in ongoing regular physical activity regardless of level of disability.[5]
Strength Training
Strong Recommendation
- Progressive resistance training should be offered to those with reduced strength in their arms or legs. [5]
Circuit Class
Van de Port et al (2012) found that task oriented circuit training in patients with mild to moderate disability after stroke is safe and as effective as an individually tailored face to face treatment in the first six months after stroke but was not superior to usual care in terms of self reported mobility according to the mobility domain of the stroke impact scale. Circuit training did prove more effective in terms of walking speed, stair walking, and walking distance, though differences were small; 9 cm/s for walking speed and 20 m for walking distance, respectively. [47] There is also growing evidence that circuit training is effective at improving the walking competency of patients in the chronic phase of stroke. The benefit of Circuit Training is Another important aspect of the task oriented circuit training is that it is offered in groups ranging from two to eight patients, lowering ratios of staff to patients and therefore a possible more cost effective treatment. [48]
Hydrotherapy
Immersion in water can enhance the treatment of neurologically impaired individuals with both therapeutic, physchological and social benefits. Hydrotherapy is the term used for exercise in warm water and is a popular treatment for patients with neurologic and musculoskeletal conditions and is defined by the Hydrotherapy Association of Chartered Physiotherapists Guidance on Good Practice in Hydrotherapy as a therapy programme using the properties of water, designed by a suitably qualified physiotherapist, to improve function, ideally in a purpose-built and suitably heated hydrotherapy pool [49] [50].
Merholz et al (2011) found insufficient evidence to conclude that water based activities for people after stroke are effective for reducing disability but likewise found insufficient evidence to conclude that water-based exercises are ineffective or even harmful [51]. A recent RCT showed positive results and a large improvement in high level balance and walking function after a 4 week hydrotherapy programme. Further research is required which need to focus on higher quand larger RCTs to evaluate the effectiveness of water-based exercises for people after stroke.[52]
Electrotherapy
Weak Recommendation
Spasticity Management
There is considerable debate on the definition, physiological nature and importance of spasticity. Spasticity can cause discomfort or pain for the and can be associated with activity limitation. Spasticity is common, especially in a non-functional arm with close association between spasticity and other impairments of arm function and mobility. [7]
Stretch
Stretch may be applied in a number of ways during neurological rehabilitation to achieve different effects. The types of stretching used include; Fast / Quick, Prolonged and Maintained. Currently the evidence for stretching in stroke rehabilitation is weak in relation to its use in spasticity managament.
Weak Recommendation AGAINST
- Routine use of stretch to reduce spasticity is not recommended.
- Adjunct therapies to Botulinum toxinum A such as electrical stimulation, casting, taping and stretching may be used to reduce spasticity. [5]
Botulinum Toxin
Medication injected into overactive spastic muscles to locally block spasticity. BoNT-A injections contribute to improved walking function in combination with physiotherapy. Similarly, the use of BoNT-A for improving hand function is effective in combination with occupational therapy. Injections in the submandibular glands are effective to reduce drooling. BoNT-Aadministration improves spasticity, range of movement and ease of care (i.e. passive function) and clinical goal attainment but not activity-level function (i.e. active function). [7]
Weak Recommendation
- Botulinum Toxin A in addition to rehabilitation therapy may be used to reduce upper limb spasticity but is unlikely to improve functional activity or motor function. [54]
- Botulinum Toxin A in addition to rehabilitation therapy may be useful for improving muscle tone in patients with lower limb spasticity but is unlikely to improve motor function or walking. [5]
Contracture Management
A muscle contracture is a permanent shortening of a muscle or joint. It is usually in response to prolonged hypertonic spasticity in a concentrated muscle area. Contractures are not uncommon in limbs affected by spasticity. Contractures can impede activities such as washing or putting on clothes, and may also be uncomfortable or painful and limit the ability to sit in a wheelchair or mobilise.A Systematic Review to determine whether stretch increases joint mobility in people with existing contractures or those at risk of developing contractures provides moderate to high quality evidence that stretch, whether passive or through the means of splint or seriel casting, does not have a clinically important effect on joint mobility in people with neurological conditions.
Strong Recommendation AGAINST
Practice Statement
Consensus-based Recommendations
Fatigue Management
Fatigue is common complaint post-stroke, and is evident even in those individuals who have made an otherwise complete recovery. Over 40% of long-term stroke survivors report ongoing issues with fatigue which impact on their daily living activities with lack of energy and/or an increased need to rest every day, as the main characteristics which can be brought on by both mental and physical activity. Fatigue has also been associated with depression, and may be a predictor of shorter survival. [7] [5] Management strategies include the identification of triggers and re-energisers, environmental modifications and lifestyle changes, scheduling and pacing, cognitive strategies to reduce mental effort, and psychological support to address mood, stress and adjustment. [7] [57]
Strong Recommendation
- Individuals with stroke who are medically stable but who report fatigue should be offered an assessment for mental and physical factors that may be contributing, particularly when engagement with rehabilitation or quality of life is affected. [7]
Practice Statement
Consensus-based Recommendations
- Therapy for stroke survivors with fatigue should be organised for periods of the day when they are most alert.
- Information and education about fatigue should be provided to individuals with Stroke and their Families/Carers. [7]
- Potential modifying factors for fatigue should be considered including avoiding sedating drugs and alcohol, screening for sleep- related breathing disorders and depression
- While there is insufficient evidence to guide practice, possible interventions could include exercise and improving sleep hygiene [5]
References
- ↑ 1.001.011.021.031.041.051.061.071.081.091.10 Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, Kwakkel G. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PloS one. 2014 Feb 4;9(2):e87987.
- ↑ De Jong L.D., Nieuwboer A., & Aufdemkampe, G. (2006). Contracture preventive positioning of the hemiplegic arm in subacute stroke patients: a pilot randomized controlled trial. Clinical Rehabilitation, 20: 656-667.
- ↑ Ada L., Goddard E., McCully J., & Bampton J. (2005). Thirty minutes of positioning reduces the development of shoulder external rotation contracture after stroke: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation, 86(2): 230-34.
- ↑ 4.04.1 Scottish Intercollegiate Guidelines Network. Management of Patients With Stroke: Rehabilitation, Prevention and Management of Complications, and Discharge Planning: a National Clinical Guideline. (2010)
- ↑ 5.005.015.025.035.045.055.065.075.085.095.105.115.125.135.145.155.165.175.185.195.205.215.225.235.245.255.265.275.28 Stroke Foundation. DRAFT Clinical Guidelines for Stroke Management 2017. Summary of Recommendations.
- ↑ 6.06.1 Clinical Guidelines for Stroke Management A Quick Guide for Physiotherapy. National Stroke Foundation, Australia, 2010.
- ↑ 7.007.017.027.037.047.057.067.077.087.097.107.117.127.137.147.157.167.177.187.197.207.21 Intercollegiate Stroke Working Party. National Clinical Guideline for Stroke Fifth Edition. 2016
- ↑ 8.08.18.28.38.48.5 Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, Kwakkel G. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PloS one. 2014 Feb 4;9(2):e87987.
- ↑ 9.09.1 Cabanas-Valdes R, Cuchi GU & Bagur-Calafat C, 2013. Trunk training exercises approaches for improving trunk performance and functional sitting balance in patients with stroke: a systematic review. Neurorehabilitation, 33, 575-92.
- ↑ 10.010.1 Pollock A, Gray C, Culham E, Durward Brian R, et al, 2014a. Interventions for improving sit-to-stand ability following stroke. Cochrane Database of Systematic Reviews, CD007232.
- ↑ Bang DH, Cho HS. Effect of body awareness training on balance and walking ability in chronic stroke patients: a randomized controlled trial. Journal of physical therapy science. 2016;28(1):198-201.
- ↑ 12.012.1 van Duijnhoven HJ, Heeren A, Peters MA, Veerbeek JM, Kwakkel G, Geurts AC, Weerdesteyn V. Effects of Exercise Therapy on Balance Capacity in Chronic Stroke. Stroke. 2016 Oct 1;47(10):2603-10.
- ↑ Stanton R, Ada L, Dean CM, Preston E. Biofeedback improves activities of the lower limb after stroke: a systematic review. Journal of physiotherapy. 2011 Dec 31;57(3):145-55.
- ↑ Tyson SF & Kent RM, 2013. Effects of an ankle-foot orthosis on balance and walking after stroke: a systematic review and pooled meta-analysis. Archives of Physical Medicine & Rehabilitation, 94, 1377-85.
- ↑ English C, Hillier SL. Circuit class therapy for improving mobility after stroke. The Cochrane Library. 2010 Jan 1.
- ↑ 16.016.1 Mehrholz J, Pohl M, Elsner B. Treadmill training and body weight support for walking after stroke. The Cochrane Library. 2014 Jan 1.
- ↑ Corbetta D, Imeri F, Gatti R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: a systematic review. Journal of physiotherapy. 2015 Jul 31;61(3):117-24.
- ↑ Rodrigues-Baroni JM, Nascimento LR, Ada L, Teixeira-Salmela LF. Walking training associated with virtual reality-based training increases walking speed of individuals with chronic stroke: systematic review with meta-analysis. Brazilian journal of physical therapy. 2014 Dec;18(6):502-12.
- ↑ Mehrholz J, Elsner B, Werner C, Kugler J, Pohl M. Electromechanical-assisted training for walking after stroke. Stroke. 2013 Oct 1;44(10):e127-8.
- ↑ Stanton R, Ada L, Dean CM, Preston E. Biofeedback improves activities of the lower limb after stroke: a systematic review. Journal of physiotherapy. 2011 Dec 31;57(3):145-55.
- ↑ Nascimento LR, de Oliveira CQ, Ada L, Michaelsen SM, Teixeira-Salmela LF. Walking training with cueing of cadence improves walking speed and stride length after stroke more than walking training alone: a systematic review. Journal of physiotherapy. 2015 Jan 31;61(1):10-5.
- ↑ Howlett OA, Lannin NA, Ada L, McKinstry C. Functional electrical stimulation improves activity after stroke: a systematic review with meta-analysis. Archives of physical medicine and rehabilitation. 2015 May 31;96(5):934-43.
- ↑ 23.023.1 Shepherd R, Carr J. Treadmill walking in neurorehabilitation. Neurorehabilitation and Neural Repair. 1999 Sep;13(3):171-3.
- ↑ Mehrholz J, Pohl M. Electromechanical-assisted gait training after stroke: a systematic review comparing end-effector and exoskeleton devices. Journal of rehabilitation medicine. 2012 Mar 5;44(3):193-9.
- ↑ Prassas S, Thaut M, McIntosh G, Rice R. Effect of auditory rhythmic cuing on gait kinematic parameters of stroke patients. Gait & Posture. 1997 Dec 1;6(3):218-23.
- ↑ 26.026.126.2 Nascimento LR, de Oliveira CQ, Ada L, Michaelsen SM, Teixeira-Salmela LF. Walking training with cueing of cadence improves walking speed and stride length after stroke more than walking training alone: a systematic review. Journal of physiotherapy. 2015 Jan 31;61(1):10-5.
- ↑ Barclay RE, Stevenson TJ, Poluha W, Ripat J, Nett C, Srikesavan CS. Interventions for improving community ambulation in individuals with stroke. The Cochrane Library. 2015 Jan 1.
- ↑ Natasha Lannin (2011). Orthotics in Neurorehabilitation. NeuroRehabilitation 28, 15–16
- ↑ 29.029.1 Leonard et al (1989). Prosthetics, Orthotics and Assistive Devices 1. General Concepts. Arch. Pays. Med. Rehab. 70, S195-S201
- ↑ Tyson SF, Kent RM. The effect of upper limb orthotics after stroke: a systematic review. NeuroRehabilitation. 2011 Jan 1;28(1):29-36.
- ↑ Momosaki R, Abo M, Watanabe S, Kakuda W, Yamada N, Kinoshita S. effects of ankle–foot orthoses on functional recovery after stroke: a propensity score analysis based on Japan rehabilitation database. PloS one. 2015 Apr 2;10(4):e0122688.
- ↑ Van Delden AE, Peper CE, Beek PJ, Kwakkel G. Unilateral versus bilateral upper limb exercise therapy after stroke: a systematic review. Journal of rehabilitation medicine. 2012 Feb 5;44(2):106-17.
- ↑ Coupar F, Pollock A, Van Wijck F, Morris J, Langhorne P. Simultaneous bilateral training for improving arm function after stroke. The Cochrane Library. 2010 Apr 14.
- ↑ Morris DM, Taub E, Mark VW. Constraint-induced movement therapy: characterizing the intervention protocol. Eura Medicophys. 2006;42(3):257–68
- ↑ Taub, E.,Uswatte, G. Constraint-induced movement therapy: answers and questions after two decades of research. 2006 NeuroRehabilitation, 21(2), 93-95.
- ↑ Corbetta D, Sirtori V, Castellini G, Moja L, Gatti R. Constraint‐induced movement therapy for upper extremities in people with stroke. The Cochrane Library. 2015.
- ↑ Wee SK, Hughes AM, Warner M, Burridge JH. Trunk restraint to promote upper extremity recovery in stroke patients: a systematic review and meta-analysis. Neurorehabilitation and neural repair. 2014 Sep;28(7):660-77.
- ↑ Howlett OA, Lannin NA, Ada L, McKinstry C. Functional electrical stimulation improves activity after stroke: a systematic review with meta-analysis. Archives of physical medicine and rehabilitation. 2015 May 31;96(5):934-43.
- ↑ Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B. Electromechanical and robot‐assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. The Cochrane Library. 2015.
- ↑ Laver K, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation. Stroke. 2012 Feb 1;43(2):e20-1.
- ↑ Thieme H, Mehrholz J, Pohl M, Behrens J, Dohle C. Mirror therapy for improving motor function after stroke. Stroke. 2013 Jan 1;44(1):e1-2.
- ↑ Liu H, Song LP, Zhang T. Mental practice combined with physical practice to enhance hand recovery in stroke patients. Behavioural neurology. 2014 Nov 9;2014.
- ↑ Braun SM, Beurskens AJ, Borm PJ, Schack T, Wade DT. The effects of mental practice in stroke rehabilitation: a systematic review. Archives of physical medicine and rehabilitation. 2006 Jun 30;87(6):842-52.
- ↑ Page SJ, Peters H. Mental practice: applying motor PRACTICE and neuroplasticity principles to increase upper extremity function. Stroke. 2014;45(11):3454-60.
- ↑ Liu H, Song LP, Zhang T. Mental practice combined with physical practice to enhance hand recovery in stroke patients. Behavioural neurology. 2014 Nov 9;2014.
- ↑ Tyson SF, Kent RM. The effect of upper limb orthotics after stroke: a systematic review. NeuroRehabilitation. 2011 Jan 1;28(1):29-36.
- ↑ van de Port IG, Wevers LE, Lindeman E, Kwakkel G. Effects of circuit training as alternative to usual physiotherapy after stroke: randomised controlled trial. Bmj. 2012 May 10;344:e2672.
- ↑ English C, Hillier SL. Circuit class therapy for improving mobility after stroke. The Cochrane Library. 2010 Jan 1.
- ↑ Hydrotherapy Association of Chartered Physiotherapists, HACP. Guidance on good practice in hydrotherapy. www.csp.org.uk accessed 14 May 2017
- ↑ Hiroharu K., Kiichiro T. Effectiveness of Aquatic Exercise and Balneotherapy: A Summary of Systematic Reviews Based on Randomized Controlled Trials of Water Immersion Therapies. Journal of epidemiology 2010; Vol.20;1:2-12
- ↑ Mehrholz J, Kugler J, Pohl M. Water‐based exercises for improving activities of daily living after stroke. The Cochrane Library. 2011 Jan 1.
- ↑ Zhu Z, Cui L, Yin M, Yu Y, Zhou X, Wang H, Yan H. Hydrotherapy vs. conventional land-based exercise for improving walking and balance after stroke: a randomized controlled trial. Clinical rehabilitation. 2016 Jun 1;30(6):587-93.
- ↑ Vafadar AK, Côté JN, Archambault PS. Effectiveness of functional electrical stimulation in improving clinical outcomes in the upper arm following stroke: a systematic review and meta-analysis. BioMed research international. 2015 Jan 22;2015.
- ↑ Turner-Stokes L, Fheodoroff K, Jacinto J & Maisonobe P, 2013. Results from the Upper Limb International Spasticity Study-II (ULIS-II): A large, international, prospective cohort study investigating practice and goal attainment following treatment with botulinum toxin a in real-life clinical management. BMJ Open, 3.
- ↑ Katalinic OM, Harvey LA, Herbert RD, Moseley AM, Lannin NA, Schurr K. Stretch for the treatment and prevention of contractures. The Cochrane Library. 2010 Sep 8.
- ↑ College of Occupational Therapists andfckLRAssociation of Chartered Physiotherapists in Neurology. Splinting of the Prevention and Correction of Contractures in Adults with Neurological Dysfunction: Practice Guideline for Occupational Therapists and Physiotherapists (2015). Available at: http://www.acpin.net/Downloads/Splinting_Guidelines/Splinting_Guidelines.pdf
- ↑ Wu S, Kutlubaev Mansur A, Chun Ho-Yan Y, Cowey E, et al, 2015. Interventions for post-stroke fatigue. Cochrane Database of Systematic Reviews, CD007030.