Introduction
Phantom limb pain(PLP) is defined as “pain that is localised in the region of the removed body part” [1]. It is a poorly understood clinical phenomenon that remains the subject of intense research due to the acute and chronic nature of the condition. The incidence is reported to be as high as 60-80% in patients post amputation [2] and risk factors include chronic pre-amputation pain, post-operative surgical pain and psychological distress.
- Phantom pains often described as crushing, toes twisting, hot iron, burning, tingling, cramping, shocking, shooting, “pins & needles”
- Tends to localise to more distal phantom structures (e.g. fingers and toes)
- Prevalence in early stages 60-80%
- Independent of age in adults, gender, level or side of amputation
Phantom sensation
Individuals with amputation may also experience phantom sensation, which is different to PLP. Phantom sensation is almost universal and doesn’t correlate with pain reports. There are three types of phantom sensations:
- Kinetic (movement)
- Kinesthetic (size, shape, position)
- Exteroreceptive (touch, pressure, temperature, itch, vibration)
Onset
Onset is mostly immediate after amputation, some at a few weeks, rarely months later. One third of patients experience maximal symptoms immediately post-op and generally resolved by 100 days, a half experience pain that slowly peaks and is improved within 100 days, a quarter of patients experience a slower rise towards maximal pain[3].
Natural history
PLP tends to diminish in severity and frequency over time, with resolution over several weeks to 2 years. One study showed 72% had PLP at 8 days, 65% at 6 months, 59% at 2 years[4]. Also the duration of episodes vary. One study showed continuous PLP in 12%, days 2%, hours 37%, seconds 38%[5]), 50% had decreasing PLP with time, 50% no change or increase over time[6].
Aetiology
There are numerous theories about the causes of phantom limb pain including peripheral, central and spinal theories:
Peripheral Theories
- Remaining nerves in the stump grow to form neuromas, which generate impulses. These impulses are perceived as pain in the limb which has been removed.
- After changes in the severity of phantom limb pain were noted in different temperatures, another theory says that cooling of the nerve endings increases the rate of firing of the nerve impulses, which are perceived by the patient as phantom limb pain
Central Theories
- Melzack proposed that the body is represented in the brain by a matrix of neurons. Sensory experiences create a unique neuromatrix, which is imprinted on the brain. When the limb is removed, the neuromatrix tries to reorganise but the neurosignature remains due to the chronic pain experienced prior to the amputation. This causes phantom limb pain after amputation.
Spinal Theories
- When peripheral nerves are cut during amputation, there is a loss of sensory input from the area below the level of amputation. This reduction in neurochemicals alters the pain pathway in the dorsal horn
Drivers and treatment options
When PLP is present it is important to establish the principle driver(s). These may be centrally driven adaptation, peripheral sensitisation, mental state or social concerns, and musculoskeletal factors. Treatment should target these drivers.
| Treatment options | Drivers | |
|---|---|---|
| Central Adaptation |
Mental imagery (also included within GMI) |
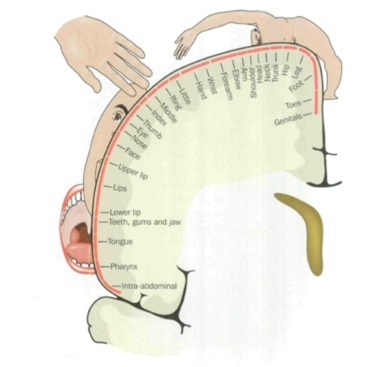
PLP appears to coexist with a reorganisation of the cortical map. For example, in upper limb amputees, the greater the shift of the mouth and face representation into the deafferented hand and arm amputation zone, the greater the PLP. Stimulation of facial muscles, including mastication or eye movements, will then elicit PLP. In lower limb amputations this phenomenon can manifest in the migration of the representation areas for the bladder, bowel and genitals into the amputation zone. Again, stimulation of these organs will elicit PLP. |
| Peripheral Sensitisation |
Irritant management with attention to excluding differential diagnosis, poor wound dressings, stump oedema. |
Nociceptive input from the residual limb appears to correlate with the level of PLP. The dorsal root ganglion can amplify discharge from the residual limb or cross-excite neighbouring neurons. Increased circulating epinephrine resulting from sympathetic discharge will also trigger or exacerbate neuronal activity. Such sympathetic discharge can result from emotional distress, and may also be due to temperature or inflammation. Continued nociceptive stimulation will cause the peripheral nervous system to become more efficient at transmitting these signals and in turn contribute to neuropathic excitation. |
| Psychological and Social Factors |
Education |
Is pain influenced by memory of the incident, memory of pain proceeding the amputation, mood state, social concerns or sleep pattern? Circulating epinephrine resulting from emotional distress can contribute to the sensitisation of the peripheral nervous system. |
| Musculoskeletal (MSK) Factors |
Joint ROM / muscular |
Joint dysfunction and MSK referral can contribute to the presence of PLP. In addition, prosthetic use significantly aids resolution of PLP, especially with the upper limb. Preparatory work to ensure the maintenance of joint range, normal symmetrical movement and proximal stabilisation will aid prosthetic fitting and successful use, This will potentially enhance the beneficial effect of limb wearing upon PLP. |
Assessment and decision-making
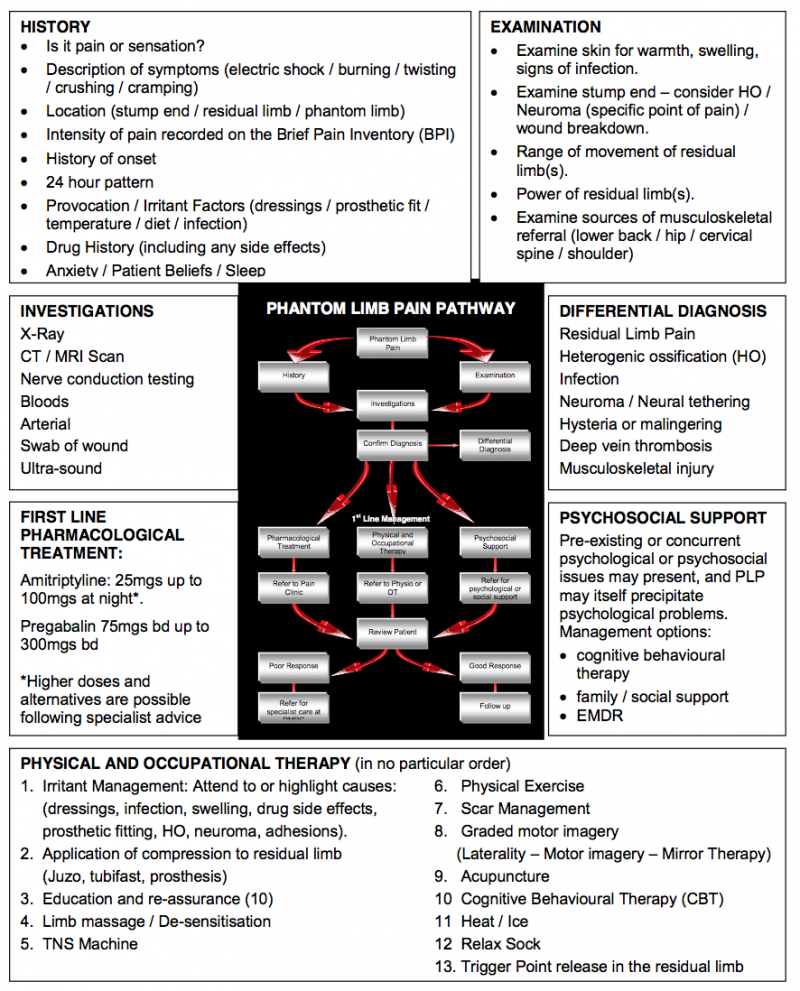
The image below shows an assessment approach, which may help clinicians to determine the correct course of action required with a patient with PLP. The assessment must commence by accurately identifying that PLP is indeed the issue. Knowledge of the different characteristics of each pain presentation will help the clinician to establish this from an assessment of their history:
An aid to clinical reasoning in phantom limb pain[7]
Simply discriminating between residual limb pain (RLP) and PLP is more complex than it appears. Both often coexist and RLP may provoke PLP. Eliminating the causes of RLP is therefore the priority as this will resolve or lessen PLP which is respondent to peripheral aggravators. It also shows the degree to which central factors may have an ongoing influence.
Immediate post-amputation management demands early effective analgesia and adjunctive measures include managing oedema using elastic stump socks, semi-rigid dressings and rigid plaster casts. Post-acute management requires attention to both intrinsic and extrinsic causes of RLP.
Extrinsic RLP will result from complications of wound healing and so infection must be excluded. Tissue load and sheering forces placed on the limb due to a poor prosthetic fit will also evoke pain. A prosthetic review will improve fit and enable sensitised structures to be offloaded. Scar formation can also cause pain, particularly where there is nerve entrapment, or adhesions reducing the mobility of soft tissues. In either case, scar management using soft tissue massage and moisturiser is recommended; silicone treatment can also be added if required. Besides improving tissue mobility, massage can be used to desensitise the residual limb. Intrinsic causes of RLP can include ischaemia, joint dysfunction proximal to the residual limb, stress fracture, osteomyelitis and wound dehiscence. Occasionally where the bone has been improperly trimmed or formation of bone in extraskeletal soft tissue has occurred, then pain may result in high-pressure areas. Investigations will be required and revision surgery may be considered; alternatively, prosthetic adjustment can be used to unload pressure areas.
Neuroma is the most common cause of intrinsic RLP. Ectopic discharge may evoke a neuropathic response causing PLP. Neuroma formation after amputation is normal, but when it becomes sensitised to mechanical or chemical stimuli, often exacerbated by entrapment, then problems ensue[8][9]. Pain is intermittent and variable, but diagnosis is confirmed by a specific site of tenderness on palpation, which can be confirmed with an injection of local anaesthetic into the site. Surgical referral can be considered, but massage, vibration, acupuncture and transcutaneous electrical nerve stimulation (TENS) may also effectively desensitise the area[10][11]. It is also work excluding muscle tension / spasm as a cause by assessing local and trigger points within the soft tissue.
Combining physical and occupational therapy with a cognitive understanding of the condition will amplify the effects of treatment[12][13]. We should aim to equip and empower the patient, informing them about their condition and how they can take control while seeking to alter destructive or erroneous beliefs and actions. Common self-treatment strategies can include wearing an elastic stump sock to minimize volume changes in the residual limb, stump massage, mental imagery of the phantom limb and taking physical exercise.
Visualisation of limb movement and prosthetic use can reduce PLP, this is especially the case with upper limb amputees[8][14]. Joint dysfunction proximal to the residual limb and prosthetic fit will however undermine this effect. Good prosthetic use is vital. Normalising the gait pattern is, in part, due to prosthetic fit and alignment. It is also dependent on good proprioception, correct motor patterning and symmetrical movement control enabling dissociation of movement between trunk and limb. In turn, the residual limb(s), trunk and spinal segments must have sufficient range and control of movement to achieve a symmetrical gait pattern. Where limb wearing is not possible, the therapist should engage their creativity to seek ways of simulating visual and even motor stimuli in order to mimic the use of the limb.
Mirror therapy
Mirror therapy is a therapeutic intervention, which has been shown to affect motor and sensory processes through the relative dominance of the visual input it provides. The effect is created by viewing a reflection of the intact limb, through a mirror placed where the amputated limb would have existed. Most of the evidence for this intervention comes from case studies and anecdotal data with only a couple of well controlled studies[15]. Moseley argued that while mirrored movements may expose the cortex to sensory and motor input, the therapeutic effect is magnified if cortical networks are gradually activated using limb recognition, motor imagery and finally, mirrored movement. This sequence of cortical exposure has become known as graded motor imagery[13][15]. Clinicians wishing to add this programme to their treatment repertoire can find resources at
UK military pain management system encourages the use of antineurppathic medication such as pregabalin and amitriptyline as early as possible. First-line treatment is a trial of up to 300 mg twice daily of pregabalin and up to 150 mg of amitriptyline at night. If pregabalin is insufficient, or depression is a problem, duloxetine may be used. Opioids are of variable help. It may be that tapentadol will prove to be beneficial, but it is too early to say clearly. While pharmacological agents can be of use, the way they are used is even more important. Pharmacological agents are not going to remove all pain. What really matters is that the agents enable the patient to ‘do more’. In this way they can be likened to the old confectionary advertisement that suggested it allows you to ‘work, rest and play’; the point being if the pharmacological agents do not have this action there is no point taking them. Often a good starting point is to enable good sleep. You can always have a good night after a bad day, but never a good day after a bad night.
A note on medication
Resources