Clinically Relevant Anatomy
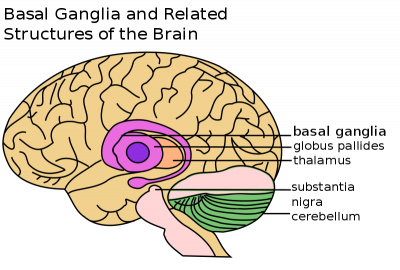

Definition
Fahr’s syndrome is also known as Fahr’s disease, familial idiopathic basal ganglia calcification and primary familial brain calcification. It is a rare neurological disorder characterized by bilateral calcifications of areas in the brain including[2][3]:
- basal ganglia (most commonly the globus pallidus)
- cerebellum (most commonly the dentate nucleus)
- thalamus
- hippocampus
- cerebral cortex
Calcifications are hypothesized to be due to lipid deposition and demyelination[4]. The presentation of an individual with Fahr’s disease can vary greatly with some remaining asymptomatic despite receiving imaging confirmation of calcification. In more severe cases individuals will present first and most prominently with extrapyramidal symptoms[5][6]. Further symptoms may include: progressive psychosis, cognitive impairment, dementia, gait disturbance and sensory changes[7]. Fahr’s syndrome can present at any age, but is typically first diagnosed in individuals between 40-60 years old[2][4][6][7].
Pathological Process/Prognosis
Etiology
Fahr’s syndrome is familial and inherited, with autosomal dominant cases making up 60% of diagnoses[7]. Some research has shown that fewer cases may be inherited in an autosomal recessive fashion[3]. There are also several other factors that could lead to brain calcification which include: endocrinopathies, vasculitis, mitochondrial disorders, infections, other inherited disorders, radiation, chemotherapy and carbon monoxide poisoning[7].
Prognosis
Prognosis differs from person to person and thus is hard to predict[2]. Fahr’s syndrome is a progressive disease with no known cure and no specific treatments at this time[7]. Due to Fahr’s progressive and degenerative features individuals will often lose previously acquired skills and motor control, which can lead to death[8]. There is no direct correlation between the amount of calcium deposits that are seen in the brain and the degree of neurological impairments displayed by an individual with the disease[2][7].
Clinical Presentation
Lesions in the basal ganglia can cause patients to present with different motor deficits. These include slowness of movement, involuntary extra movement and alterations in posture and muscle tone[9]. Therefore patients with basal ganglia involvement can present on a continuum of motor behaviour from severely limited as seen in the final stages of [9]. In “Fahr’s Disease Registry”, the most common symptoms were movement disorders, in particular parkinsonism, which affects more than half of patients[3].
| Associated Movement Disorders | |
|---|---|
| Dystonia | A movement disorder that causes [10]. Dystonia can affect one or several regions of the body[11]. There is presently no cure for dystonia, but the goal is to help decrease the severity of muscle spasms, pain and awkward postures to improve overall quality of life[12]. |
| Athetosis | A movement disorder characterised by slow, smooth, sinuous, writhing movements, also described as “wormlike movements”[13]. More common in the distal upper extremities, but also prevalent in other areas of the body such as face, trunk, neck and tongue[14]. Pure Athetosis is uncommon as it usually presents with a combination of spasticity, tonic spasms or chorea[15]. |
| Chorea | Abnormal movement involving involuntary, irregular, purposeless, non-rhythmic, abrupt, rapid and unsustained, that can flow from one area of the body to another. These movements can vary in amplitude, small movements of the fingers to flailing of limb movements, referred to as ballism[16]. Patients are at an increased falls risk due to impairments in balance, strength and increased fatigue. Musculoskeletal and respiratory changes can result in physical deconditioning and contribute to decreased participation in daily activities and social participation[17]. |
| [18]. |
|
| Tremor | Involuntary shaking movements. Tremor can be seen in the extremities, usually as a resting tremor the presents when a patient is at rest, or in the head and trunk, when the patient is trying to hold an upright posture[19]. A resting tremor can eventually progress to an action tremor, which is tremor with movement. Although the pathophysiology is slightly different from Fahr’s, Parkinson’s patients tend to exhibit a mild tremor first on only one side of the body[19]; there is not enough data to decisively say if this it true for Fahr’s patients. Tremors tend to worsen with stress, anxiety or an excited emotional state[19]. Particularly in later stages, tremors interfere with the ability to perform functional activities, especially fine motor tasks such as picking up or holding objects. |
| Rigidity | Increased resistance to passive movement that is not affected by speed or amplitude of motion[19]. There are two types: lead pipe – which is constant throughout range – and cogwheel – which is jerky with tension felt intermittently throughout a movement[19]. Rigidity affects a patient’s ability to move and therefore independently carry out activities of daily living (ADLs). In many patients, rigidity can be increased by stress or active movements. |
| Hypomimia | The reduced ability to portray facial expressions, both automatic and voluntary, that is often seen in Parksinon’s and Fahr’s patients. This frozen, masked expression is often incorrectly interpreted by others as depression, coldness, apathy and reduced cognition[20]. This can cause difficulty in communication and relationships, including patient-therapist relationships; studies have shown that practitioners – including physiotherapists – tend to view patients with facial masking as more depressed, less sociable and less cognitively competent[20]. Therefore it is an important component of the treatment of Fahr’s disease to not allow oneself to form negative preconceptions about a client based on a symptom they cannot control. |
| Gait | Affected by Fahr’s disease similarly to Parkinson’s disease. Fahr’s patients can exhibit unsteadiness, clumsiness, a shuffling gait, or freezing of gait[21]. Hypokinesia, which is a generalized slowness of movement, can also manifest in gait as an increased time to initiate and execute movement as well as decreased force generation[19]. |
| Hypokinesia | A generalized slowness of movement. It can also manifest in gait as an increased time to initiate and execute movement as well as decreased force generation[19]. |
Neuropsychiatric and Other Symptoms
Due to the heterogeneity of brain calcifications and associated impairments, it is common to see a wide variety of symptoms other than movement disorders[5]. These include epileptic seizures, dysarthria, mood disorders, problems with cognition (memory and concentration), behaviour/personality changes, psychosis and dementia[5][22]. While some of these symptoms may not be directly treatable with physiotherapy interventions, they do affect considerations for treatment and are important to keep in mind when planning for patient care. Included below are links to resources within Physiopedia and external sources that provide more information regarding these symptoms.
Diagnostic Procedures
| Diagnostic criteria |
|---|
| 1. Genetic abnormality and family history inheritance of autosomal dominant trait or autosomal recessive trait[7][23] |
| 2. Bilateral calcification in Basal Ganglia visible on neuroimaging[5][23] |
| 3. Exclude secondary causes of Fahr syndrome and biochemical abnormalities (infection, traumatic injury, metabolic, toxic)[5][23] |
| 4. Progressive neurological dysfunction involving movement and psychiatric disorders[5][7][22][23] |
Neuroimaging
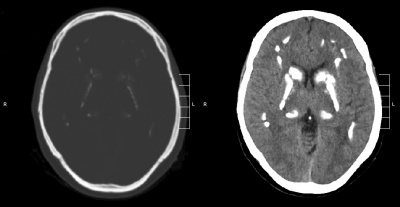

- CT – used to assess the location and severity of cerebral calcification[7][23]
- MRI – generally less useful than CT, may be used to locate calcification in the brain, but appearance varies depending on stage of disease and the amount of calcification[7][23]
- X-ray – may be used to locate symmetrical calcium clusters lateral to midline[7][23]
Tests
- Molecular Genetic testing – assess presence of mutations or deletions in SLC20A2 or PDGFRB gene[5]
- Urine and blood analysis – assess calcium metabolism and presence of heavy metals in the body[5]
- CSF analysis – assess possible infectious or autoimmune cause of brain calcification[5]
Physical Exam
- Complete Neurological Exam
- Assess Strength,Tone and Spasticity
- Assess Balance, Gait, Function Assessment (see Outcome Measures below)
Other Assessments
- Mental Health Screen
- Memory and Cognitive Testing
Outcome Measures
Medical Management
There is no cure or standard treatment plan for Fahr’s syndrome. Symptoms and disease presentation are treated on an individual basis. Pharmacologic treatment can be helping in alleviating seizures, headaches and some psychiatric symptoms[23]. There are no known studies on whether dementia medications are helpful in treating associated symptoms in Fahr’s Syndrome. Parkinson-like symptoms are generally non-responsive to Levodopa[23].
Physiotherapy Intervention
Treatment Note
Due to the lack of literature on Fahr’s disease and the similarity of symptoms with Parkinson’s, Huntington’s and other neurological conditions, therapists should use their clinical judgement in combination with evidence about the treatment of the overlapping symptoms in similar populations. Recommendations for treatment include a focus on function, participation and exercise capacity, as well as preventing or slowing the progression of secondary impairments such as muscle weakness[25].
| Treatment Goals May Include |
|---|
| 1. Increase and/or maintain range of motion, prevent contractures |
| 2. Strengthen weakened muscles that may be underutilized |
| 3. Improvement and maintenance of postural stability in static postures and during mobility |
| 4. Gait retraining and/or falls prevention |
| 5. Symptom management |
- Maintaining Range of Motion and Flexibility
- Range of motion exercises, passive stretching and facilitated stretching can help maintain tissue extensibility and physical functioning[25]. These exercises should ideally be done 5-7 days per week, but at least 2-3 days, with 3-4 reps of at least 15-60 seconds per stretch[26]. Stretching can be combined with [26].
- Note for patients with spasticity, therapist should use constant pressure over bony prominences and avoid direct pressure on spastic muscles. Limb movement out of spastic position should be slow, repeated motions[25].
- Serial casts may be used for patients at risk of contractures and deformity[25].
- Note for patients with dystonia, braces may be helpful in preventing contractures. However, they tend to be poorly tolerated and are only really used for writer’s cramp to enable the hand to be used more effectively and comfortably[27]
- Strengthening Underutilized Muscles
- A general conditioning program is beneficial for most patients with neurological disorders to maintain strength and function[26].
- Include the principles of overload, specificity, cross training and reversibility in designing a program. Note with specificity, it is best to attempt to create exercises that will carry over into the client’s daily life, for example following an isometric protocol will not guarantee effects carry over to improved dynamic performance which is more applicable to ADLs[25].
- Muscles that commonly become weak in neurological populations include antigravity extensor muscles[26].
- Exercise machines, in contrast to free weights, may be safer for patients with more progressed motor symptoms since movements are more controlled[26].
- Improving Postural Instability
- Instruct patient in correct sitting posture, using appropriate vertical cuing such as lines on the wall, and the clinician exhibiting appropriate upright posture[25]
- Patients with basal ganglia dysfunction are recommended to practice [19]
- Progress activities from wide to narrow base of support, static to dynamic, low to higher levels of cognition – single vs. dual task, also increasing degrees of freedom available[25]
- Patients with basal ganglia dysfunction are encouraged to incorporate task specific balance training, specifically during functional activities such as transfers, reaching, and using stairs[19]
- Gait retraining/Falls Prevention
- For patients with basal ganglia dysfunction, auditory cueing may assist with step timing, for example counting, stepping to a metronome or music[19]. In particular, common strategies to improve Parkinsonian symptoms such as the regularity of gait and gait freezing, include visual cues like lasers attached to a walker or cane[26]. Individual studies have also shown that participation in other activities, such as dance and high-speed cycling, can potentially improve gait[28].
- Provision of assistive devices when appropriate to improve function[26]
- Educate patient on safety awareness, including how to get up after a fall and decreasing clutter in their living space
- Assessment for orthotic device may be required (for example ankle dystonia)[17]
- For hyperkinetic disorders, protective gear such as helmets, pads may be required if at high risk for falls[17]
- Symptom Management
- Relaxation techniques, such as deep breathing, are beneficial because patients with neurological conditions such as Fahr’s disease tend to experience a lot of stress and anxiety. Relaxation has documented benefits including elevated energy levels and a greater perception of control[25].
- For hyperkinetic disorders, deep brain stimulation. Which involves a surgically implanted device that sends electrical impulses to the brain to help control movement[29].
- Soft tissue release is beneficial for patients experiencing dystonia and spasticity[30][31]. Case studies have also shown benefits of massage for Parkinson’s symptoms, including gait, range of motion, pain and upper limb function[32][33].
- Sensory stimulation for patients with basal ganglia dysfunction. Note that for patients living with dystonia, many will develop sensory tricks on their own that help to improve dystonic posture, for example touching specific parts of the face. The benefits of these tricks are transient in nature and may have less effect during the later stages of the disease[34],
- There is some evidence that whole body vibration (WBV) may improve gait, tremor, rigidity, proprioception and balance in patients with neurological diseases, including Parkinson’s[26]. However, systematic reviews have concluded that more high-quality research is needed[26].
Resources for Patients
References
- ↑ John Henkel. Structure of the basal ganglia, including thalamus, globus paladus, substantia nigra, and cerebellum [image on the Internet]. Food and Drug Administration, 23 March 2009, 17:14 (UTC). Available from: https://commons.wikimedia.org/wiki/File:Basal_Ganglia_and_Related_Structures.svg
- ↑ 2.02.12.22.3 National Institute of Neurological Disorder and Stroke. Fahr’s Syndrome Information Page. ↑ 3.03.13.2 Calabro R, Spadaro L, Marra A, Bramanti P. Fahr’s disease presenting with dementia at onset: a case report and literature review. Behav Neurol 2014;2014,750975. ↑ 4.04.1 Murat Gülsün M, Baykız AF, Kabataş S, Belli B. Fahr’s Syndrome Three cases presenting with psychiatric signs. EJGM 2006;3(1):35-40. ↑ 5.05.15.25.35.45.55.65.75.8 Shafaq S, Aslam HM, Anwar M, Anwar S, Saleem M, Saleem A and Rehmani MAK. Fahr’s syndrome: literature review of current evidence. ORJD 2013;8-156. ↑ 6.06.1 Goyal D, Khan M, Qureshi B, Mier C, Lippmann S. Would you recognize Fahr’s Disease if you saw it? ICN 2014;11(1-2):26-28. ↑ 7.007.017.027.037.047.057.067.077.087.097.10 Radiopaedia. Fahr’s Syndrome. ↑ Fahr Too Strong Foundation. Commonly asked questions about Fahr’s Disease. ↑ 9.09.1 Kandel, ER, Schwartz, JH, and Jessell. Principles of neural science. 4th ed. New York: McGraw-Hill; 2000. p 853.
- ↑ Velickovic M, Benabou R, Brin MF. Cervical dystonia: pathophysiology and treatment options. Drugs 2001;61(13),1921-1943. ↑ Bressman SB. Dystonia genotypes, phenotypes, and classification. Adv in Neurol. 2004;94,101-107. ↑ The Dystonia Society. Generalized dystonia. ↑ Johnson RK, Goran MI, Ferrara MS, Poehlman ET. Athetosis increases resting metabolic rate in adults with cerebral palsy. Journal of the American Dietetic Association. 1996 Feb 1;96(2):145-8.
- ↑ Haines DE, Ard MD. Fundamental neuroscience: for basic and clinical applications. 3rd ed. Philadelphia: Churchill Livingstone Elsevier; 2006. p413.
- ↑ Fahn S, Jankovic J, Hallett M. Clinical overview and phenomenology of movement disorders. Principles and practice of movement disorders. 2nd ed. Philadelphia: Elsevier Saunders; 2011:P1-35.
- ↑ Micheli FE, LeWitt PA, SpringerLink (Online service). Chorea: causes and management. London: Springer London; 2014. ↑ 17.017.117.2 European Huntington’s Disease Network. 2013. Physiotherapy clinical guidelines for Huntington’s disease. ↑ O’Dwyer NJ, Ada L, Neilson PD. Spasticity and muscle contracture following stroke. Brain 1996;119:1737-1749. ↑ 19.019.119.219.319.419.519.619.719.819.9 Bilney B, Morris ME, Denisenko S. Physiotherapy for people with movement disorders arising from basal ganglia dysfunction. N Z J Physiother 2013;31(2),94-100. ↑ 20.020.1 Tickle-Degnen L, Zebrowitz LA, Ma H. Culture, gender and health care stigma: practitioners’ response to facial masking experienced by people with Parkinson’s disease. Soc Sci Med. 2011;73:95-102. doi:10.1016/j.socscimed.2011.05.008
- ↑ Stamelou M, Kojovic M, Edwards MJ, Bhatia KP. Ability to cycle despite severe freezing of gait in atypical parkinsonism in Fahr’s syndrome. Movement Disorders. 2011;26:2141-2142. ↑ 22.022.1 National Organization for Rare Disorders. Primary Familial Brain Calcification Information Page.
↑ 23.023.123.223.323.423.523.623.723.8 Ramos EM. Primary Familial Brain Calcification [Internet]. Advances in pediatrics. Gene Reviews.org; 2017 [cited 2018May7]. Available from: ↑ Mikhail Kalinin. Brain CT scan in a patient with Fahr’s syndrome [image on the Internet]. 5 October 2009. Available from: https://commons.wikimedia.org/wiki/File:Fahr_syndrome.gif
- ↑ 25.025.125.225.325.425.525.625.7 O’Sullivan SB, Schmitz TJ, Fulk GD. Physical rehabilitation. 6th ed. Philadelphia: F.A. Davis Company, 2014. Chapter 10.
- ↑ 26.026.126.226.326.426.526.626.726.8 O’Sullivan SB, Schmitz TJ, Fulk GD. Physical rehabilitation. 6th ed. Philadelphia: F.A. Davis Company, 2014. Chapter 18.
- ↑ Chaudhuri KR, Ondo WG, Logishetty K, Reddy P, Sherman R. Movement disorders in clinical practice. 1st ed. London: Springer; 2010. p49-65.
- ↑ Uygur M, Bellumori M, LeNoir K, Poole K, Pretzer-Aboff I, Knight CA. Immediate effects of high-speed cycling intervals on bradykinesia in Parkinson’s disease. Physiother Theor Pr 2015;31:77-82. ↑ di Biase L, Munhoz R. Deep brain stimulation for the treatment of hyperkinetic movement disorders. Expert Rev Neurother 2016;16:1067-1078. ↑ Perrault E. 2010. The effectiveness of muscle energy and massage therapy for the management of symptoms related to chronic cervical dystonia. ↑ Bavikatte G, Gaber T. Approach to spasticity in General practice. Brit J of Med Pract 2009;2:29. ↑ Donoyama N, Ohkoshi N. Effects of traditional Japanese massage therapy on various symptoms in patients with Parkinson’s disease: a case-series study. J Altern Complement Med 2012;18:294-299. doi: 10.1089/acm.2011.0148
- ↑ Donoyama N, Suoh S, Ohkoshi N. Effectiveness of Anma massage therapy in alleviating physical symptoms in outpatients with Parkinson’s disease: a before-after study. Complement Ther Clin Pract 2014;20:251-261. doi:10.1016/j.ctcp.2014.07.010
- ↑ Ramos VFML, Karp BI, Hallett M. Tricks in dystonia: ordering the complexity. J Neurol Neurosurg Psychiatry 2014;85(9):987-993.
